NEW COMBINATIONS IN LUCILIOCLINE
WITH NOTES ON SOUTH AMERICAN GNAPHALIEAE(ASTERACEAE)
[10 May 2003]
Michael O. Dillon
Department of Botany
The Field Museum, Chicago, IL 60605-2496, USA
There once was a farmer who could not distinguish between his
two horses, so he cut the tail off the white horse and left the
tail on the black one. - Billie L. Turner
[Una traducción: Una vez
había un campesino quién no podía
distinguir entre sus dos caballos,
por eso se cortó la cola del
caballo blanco y dejó la cola
en el caballo negro.]
Resumen
Una evaluación de los representantes sudamericanos de tribu Gnaphalieae Cass. ex Lecoq & Juillet. (Asteraceae) es proveído, incluyendo una discusión de puestos de subtribos. Una evaluación de la morfología en conjunto de los géneros atribuida al "Grupo de Lucilia" ha revelado la necesidad para un nuevo composición para el grupo; estos cambios han requerido el transferencia de algunas especies. Las nuevas combinaciones propuestas lo son: Luciliocline longifolia (Cuatrec. & Aristeg) M.O.Dillon & Sagást., comb. nov., L. pickeringii (A.Gray) M.O.Dillon & Sagást., comb. nov., L. piptolepis (Wedd.) M.O.Dillon & Sagást., comb. nov., L. plicatifolia (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov., L. radians (Benth) M.O.Dillon & Sagást., comb. nov., L. schultzii (Wedd). M.O.Dillon & Sagást., comb. nov., L. spathulifolia (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov., y L. turnerii ( Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov.
Abstract
A review of the South American representatives of tribe Gnaphalieae Cass. ex Lecoq & Juillet (Asteraceae) is provided, including a discussion of subtribal positions. A review of the overall morphology of the genera assigned to the "Lucilia group" revealed the need for a new circumscription for the group; these changes required the transfer of several species. The new combinations proposed are: Luciliocline longifolia (Cuatrec. & Aristeg.) M.O.Dillon & Sagást., comb. nov., L. pickeringii (A.Gray) M.O.Dillon & Sagást., comb. nov., L. piptolepis (Wedd.) M.O.Dillon & Sagást., comb. nov, L. plicatifolia (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov., L. radians (Benth.) M.O.Dillon & Sagást., comb. nov., L. schultzii (Wedd.). M.O.Dillon & Sagást., comb. nov., L. spathulifolia (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov., and L. turnerii (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov.
Introduction
This paper is based upon an oral presentation and abstract entitled, Phylogeny and Classification of the South American Inuleae (sens. ampl.) delivered at the International Compositae Conference, Royal Botanical Gardens, Kew, 24 July - 5 August 1994. That paper presented the relationships amongst genera based upon detailed observations (Dillon & Sagástegui, 1991a) and morphological cladistic analysis (Dillon, 2000). In October 2000, a modified version of this paper was posted on the ABIS website and an abbreviated version is presented here as background for new combinations.
South American Gnaphalieae
The Inuleae s. ampl. (Asteraceae) traditionally included the South American taxa now accepted in two segregate tribes, Gnaphalieae Cass. ex Lecoq & Juillet and Plucheeae Anderb. (Bremer, 1994). Worldwide, the Gnaphalieae contains 187 genera and about 1250 species (Bayer et al., in press). In South America, the Gnaphalieae consists of perhaps 22 genera and over 100 species with highest diversity in the tropical and subtropical Andean Cordillera. The majority of genera are Neotropical endemics, but some are cosmopolitan or pantropical, e.g., Achyrocline, Gamochaeta, and Pseudognaphalium; however, their greatest species diversity is in South America.
Anderberg (1991) provided a morphological cladistic analysis of the Gnaphalieae that included 72 genera, utilizing 82 characters to establish five subtribes and several putative monophyletic groups. His treatment ultimately included 146 genera or over half added intuitively. He placed South American genera into three subtribes: Cassiniinae Anderb., Gnaphaliinae (Cass.) Dumort, and Loricariinae Anderb.
Subtribe Gnaphaliinae in South America
The subtribe Gnaphaliinae (sensu Anderberg) consisted of a group of genera with worldwide distribution diagnosed as annual to perennial herbs (occasionally subshrubs), capitula with marginal pistillate florets greatly outnumbering the central hermaphroditic or staminate florets, and oblong achenes with pubescence of short, clavate trichomes. The subtribe encompassed 47 genera, with centers of diversity in Africa, Asia, and the Neotropics. No fewer than 14 genera were partially or wholly distributed in South America. Anderberg proposed several informal groups, as suggested by his cladistic analysis and intuition.
"Lucilia group"
Anderberg (1991) established the "Lucilia group" diagnosed as herbs or subshrubs, with polychromous phyllaries, pistillate florets with generally yellow corollas, hermaphroditic florets with generally purple corollas, and the corolla veins ending below the apex of the lobes. The group initially contained Lucilia, Belloa, Chevreulia, Jalcophila, Cuatrecasasiella, Berroa, and Facelis. Subsequently, Anderberg and Freire (1991) considered Gamochaeta as the sister taxon to the "Lucilia group" and Micropsis and Stuckertiella were included subsequent to their cladistic analysis. Freire (1986, 1987) published species-level cladistic analyses where both Belloa and Lucilia were combined to form an expanded Lucilia. Anderberg and Freire (1991) published another cladistic analysis where the expanded Lucilia was dismantled and its constituent taxa placed in four genera: (1) a revised Belloa (9 spp.), (2) a revised Lucilia (8 spp.), and two new genera, (3) Gamochaetopsis Anderb. & S.E.Freire (1 sp.), and (4) Luciliocline Anderb. & S.E.Freire (5 spp.).
To test the various hypotheses of relationships presented by Anderberg (1991) and Anderberg and Freire (1991), an independent morphological analysis of the "Lucilia group" was undertaken (Dillon, 2000). The analysis utilized 35 discrete characters analyzed with PAUP (Swofford, 1991). The genus Chionolaena (including Leucopholis) was used as the outgroup. The analysis yielded a basal polytomy with Chionolaena, Lucilia, Gamochaetopsis, and two additional clades. These results suggested a close potential relationship between Chionolaena and the other members of the "Lucilia group". Both Lucilia and Gamochaetopsis formed a basal grade with no unambiguous characters to distinguish them. Chionolaena (including Leucopholis), though considered by Anderberg (1991) to be a member of the Cassiniinae, shares synapomorphies with Lucilia, including biseriate trichomes (zwillingshaares; Hess, 1938) with enlarged adaxial basal cells. In Chionolaena, Gamochaetopsis, and Lucilia, the apical cells of achenial trichomes are elongate and in excess of 150 µm in length, thick-walled, acute, and never rupturing in water.
Lucilia contains the five (5) species listed by Anderberg & Freire (1991), plus a suite of reduced, caespitose Andean species, included by them in Belloa or reduced to synonymy. These include Lucilia araucana Phil., L. conoidea Wedd., L. kunthiana (DC.) Zardini, and L. nivea (Phil.) Cabrera. These are all cushion-form species most closely related to species found in eastern Argentina and Brazil. This trend in habit reduction is also evident in L. recurvata Wedd. and L. flagelliformis Wedd. The superficial resemblance of these reduced members to some Luciliocline taxa (e.g., Luciliocline longifolia, L. radiata, and L. schultzii) is purely convergence, which has lead some workers to propose non-monophyletic groupings.
Luciliocline, as interpreted here contain 13 species distributed in high-elevation habitats throughout the Andean Cordillera from Venezuela to Chile and Argentina. Greatest diversity is found in Peru where eight species have been recorded (Dillon & Sagástegui, 1991b). It is diagnosed as possessing heterogamous capitula, pappus bristles fused at the base, style branches of hermaphroditic florets rounded or obtuse, and achenes with biseriate, multicellular, capitate-glandular trichomes (Figs. 1,2,3). The species composition of Luciliocline is expanded over that of Anderberg & Freire (1991) and several species require transfers from Belloa and/or Lucilia (see below).
Belloa is here considered monotypic and restricted to austral Chile and adjacent Argentina. The lectotype (Hooker & Arnott 342, K) has been examined and, just as Hooker and Arnott (1835) stated, their material contained only old capitula lacking florets or achenes. The distinctness of this species was recognized by DeCandolle (1838) when he established Lucilia section Luciliodes based upon Lucilia chilensis Hook. & Arn. Remy (1847), in establishing Belloa chilensis (Hook. & Arn.) Remy, apparently did not examine type material, but stated that the primary difference between his Belloa and Lucilia was in the achenial trichomes, with his new genus possessing achenes "papulosa, non villosa" (Fig. 4), whereas, Lucilia possessed densely villous achenes. This difference in achenial pubescence was also stressed by Cabrera (1958).
Anderberg and Freire (1991) based their genus, Gamochaetopsis, upon Laennecia alpina Poepp. & Endl., a species originally collected in austral Chile; however, it has not been established whether the type material was examined by the authors and whether it supports the diagnosis. The genus is said to be isolated and contain three autapomorphies (p. 183): "lanate adaxial leaf surfaces, divided stereome [phyllaries], and capselas with short, clavate twin hairs." The identity of Gamochaetopsis alpina (Poepp. & Endl.) Anderb. & S.E.Freire (1991) is problematic. Its type was considered by Cabrera (1961) to be congeneric with Lucilia and stated that it could be confused vegetatively with Gamochaeta nivalis (Phil.) Cabrera, but was easily distinguished from the latter taxon by the sericeo-pubescent achenes (i.e., elongate trichomes). Cabrera (1971) considered Lucilia alpina distinct from both L. araucana and L. nivea. However, all three taxa were described as possessing sericeo-pubescent achenes.
An examination of probable type material of Laennecia alpina collected by Poeppig at the type locality [Poeppig s.n., F 878548 ex B], shows it to possess achenes with elongate trichomes identical to those found in Lucilia. The achenial trichomes described as "short, clavate twin hairs" are only known from Belloa chilensis (Hook. & Arn.) Remy (= Lucilia chilensis Hook. & Arn.), and it is possible that material referable to Belloa chilensis was taken for L. alpina. Whether Gamochaetopsis should continue to be recognized will await further study, but it may well prove congeneric with Lucilia.
Gamochaeta and Stuckertiella are sister taxa and diagnosed by truncate style branches and achenial pubescence of sessile, paired myxogenic cells. Gamochaeta contains approximately 80 species distributed primarily in the warmer regions of the New World, but with several species adventive in the Old World. Cabrera (1961) resurrected Gamochaeta and described several new species from Argentina. Drury (1970, 1971) analyzed the gnaphaloid elements in New Zealand and reduced Gamochaeta to sectional status. Gamochaeta was accepted by Holub (1976) in Flora Europaea, but Merxmüller et al. (1977) once again treated the genus as a section of Gnaphalium. Anderberg (1991) accepted Gamochaeta and stated that it had little to do with Gnaphalium (s.s.). A listing of South American species can be found in Anderberg (1991), Dillon and Sagástegui (1991a), and Freire & Iharlegui (1997). Stuckertiella has achenes identical to Gamochaeta, but is diagnosed by several autapomorphies, including involute leaf margins, 4-merous florets, and clavate pappus apices. The 4-merous floret character has been observed in Gamochaeta as well (Díaz-Piedrahíta, pers. com.). Preliminary data from molecular studies (Bayer et al., 2003) point to relationships between Gamochaeta and other members of the "Lucilia group" as discussed here.
Facelis, Berroa, and Micropsis form a clade diagnosed as annual herbs with variable capitulescences and achenes with elongate (>150 µm), myxogenic trichomes. In all these taxa, the achenial trichomes rupture through terminal pores in the apical cells when hydrated. Berroa and Micropsis are sister taxa and are distinguished from Facelis by achenial trichomes with twisted apical cells. Micropsis is further defined by autapomorphies, including paleate receptacles where the outer phyllaries enclose the pistillate florets, and achenial trichomes with unfused apical cells. Anderberg (1994) suggested that the closest relative of Micropsis was obscure, though it was most frequently associated with genera of the "Filago group." Overall morphology suggests that these three genera are best placed in the "Lucilia group" and have more obscure relationships with the "Filago group" (see below).
Chevreulia and Cuatrecasasiella are diagnosed as herbs with opposite, distichous leaves, and a persistent pappus. Chevreulia contains six species primarily in austral South America with three reaching the northern Andean Cordillera and is diagnosed by possessing fusiform achenes contracted into a filiform rostrum, barbellate pappus bristles, and biseriate, myxogenic trichomes approximately 40 µm long, with bulbous apical cells. Cuatrecasasiella is diagnosed as dioecious herbs with glabrous achenes; its two species represent closely related Northern to Southern Andean disjuncts. The relationships of these two genera are predicted to be with other Andean members of the "Lucilia group" based only on overall morphology.
The three species of Jalcophila are recorded from the northern Andes, i.e., J. colombiana Díaz & Vélez (Díaz-Piedrahita & Vélez-Nauer, 1999), J. ecuadoriense M.O.Dillon & Sagást. and J. peruviana M.O.Dillon & Sagást. (Dillon & Sagástegui, 1986). These three species are very different from Jalcophila boliviensis Anderb. & S.E.Freire (based upon Lucilia hypoleuca Wedd. ex Schultz-Bip, nom. nud.), a taxon described from southern Bolivia (Anderberg & Freire, 1990). The latter, is a highly reduced species, though not considered here as belonging to Jalcophila, since it lacks the phyllaries, pappus, achene shape, and achenial trichomes that distinguish other members of the genus. Further, it possesses a single, large capitulum on an elongate pedicle, with over 40 florets, a character quite aberrant in Jalcophila. This species is most likely a Gamochaeta, a genus diagnosed by all of the characters that distinguish this species. A population of this rare species has been discovered (J. C. Solomon 4925, MO) and should provide a source of material for molecular studies. A formal transfer to Gamochaeta is provided below to allow for the reestablishment of monophyly in Jalcophila.
"Helichrysum group"
The "Helichrysum group", with Pseudognaphalium and Achyrocline, was diagnosed by Anderberg (1991) on the basis of phyllaries with divided stereomes, yellow florets, and papillose achenial pubescence. Individual genera were defined by the ratio of pistillate to hermaphroditic florets. Subsequently, Stenophalium was placed with this group. These genera exhibit a grade of floret ratios beginning with Stenophalium, where the number of pistillate florets is reduced to one or two per capitulum and the fertile hermaphroditic florets are typically only five. Achyrocline, has 1-11(-23) pistillate florets and 1-4(-6) fertile, hermaphroditic florets. Pseudognaphalium has (25-) 40-130 pistillate florets and 5-10 (-25) functionally staminate, hermaphroditic florets. Finally, in Helichrysum, the central hermaphroditic florets far out number the pistillate florets. Should the character of the floral ratio of capitula be discarded, these weakly defined genera could be combined, with Helichrysum as the oldest valid name available. The initial result from molecular studies (Bayer et al., 2003) has identified a monophyletic group containing Anaphalis, Helichrysum, Pseudognaphalium and several other species that Anderberg (1991) considered close to Chionolaena in his Cassiniinae.
"Filago group"
The "Filago group" is an essentially Nearctic clade with African, Eurasian, and North American elements. Psilocarphus is a predominately North American genus represented in South America by one Chilean endemic (Cronquist, 1950). Anderberg (1991) and Morefield (1992) related Psilocarphus to Stylocline. Anderberg (1991) suggested that Micropsis belong in this group, an alternative position for Micropsis in the "Lucilia group" is discussed above.
Subtribe Cassiniinae Anderb. in South America
Anderberg (1991) established the subtribe Cassiniinae for a group of genera with worldwide distribution and diagnosed as follows: often dioecious or subdioecious shrubs or herbs, pholem fibers absent, phyllaries with opaque laminae, hermaphroditic florets with truncate styles possessing trichomes their abaxial surfaces, achenes usually with two vascular bundles, and pappus bristles with clavate apical cells.
Among the 16 genera placed there were Anaphalis, Antennaria, Gnaphaliothamnus, and Chionolaena. Anderberg (1991) placed the monotypic Colombian genus, Pseudoligandra Dillon & Sagást., based upon Oligandra chrysocoma Wedd. (Dillon & Sagástegui, 1990), under the synonymy of Chionolaena with a comment that its recognition would cause Chionolaena to be paraphyletic. Another segregate, Parachionolaena M.O.Dillon & Sagást., was established for Chionolaena columbiana S. F. Blake (Dillon & Sagástegui, 1991a). Freire (1993) treated this genus as congeneric with Chionolaena, along with the several species of Gnaphaliothamnus. Nesom (1990a,b) considered Gnaphaliothamnus as a distinct from, but with relationships to, Chionolaena. Later, Nesom (1994) provided a critique of the various classifications of Gnaphaliothamnus and stated that the Mexican and Central American taxa formed a monophyletic group potentially related to Chionolaena. Subsequently, Nesom (2001) stated that he could not support Gnaphaliothamnus as distinct and sank its remaining taxa into Chionolaena, transferring a suite of Mexican and Central American species. If the segregate genera proposed by Dillon and Sagástegui (1990, 1991a) are to be included in Chionolaena, the diagnosis must be modified to reflect the morphological traits not shared by the majority of the species.
Analysis of both morphological and molecular data (Bayer et al., 2003) suggest that the Cassiniinae Anderb. is polyphyletic and both Antennaria and Chionolaena (including Gnaphaliothamnus) are more closely related to genera of the "Lucilia group" as defined here.
Subtribe Loricariinae Anderb. in South America
Anderberg (1991) established the subtribes Loricariinae and Relhaniinae as sister taxa in a clade largely made up of woody genera with leaves possessing involute margins and pubescence on adaxial surfaces. The Relhaniinae contained 19 woody African genera with discolorous ray florets and rod-like achenes. The Loricariinae was diagnosed as compact, often dioecious shrubs without fibers in the phloem, crowded leaves with adaxial pubescence, achenes with more than two vascular bundles, and dimorphic pappus (i.e., apical cells of bristles acute in the pistillate florets and clavate in the hermaphroditic florets). The subtribe included Pterygopappus (Tasmania), Psychrophyton (New Zealand), and two Andean endemics, Loricaria and Mniodes, in the original cladistic analysis. Later, Anderberg added Raouliopsis (Colombian endemic) and Sinoleontopodium (China) to his group, a posteriori. The greatly condensed and compacted leafy stems of Mniodes and Raouliopsis are similar to highly reduced Luciliocline species. Authentic material of Sinoleontopodium has not been examined, but the generic description could refer to a shrubby species of Anaphalis, a genus common to the Himalayas. Preliminary results from molecular studies (Bayer et al., 2003) suggest that the Loricariinae may be an artificial construct.
New Combinations
Gamochaeta bolivensis (Anderb. & S.E.Freire) M.O.Dillon & Sagást., comb. nov.
Jalcophila bolivensis Anderb. & S.E.Freire, Brittonia 42: 139. 1990. TYPE: (based upon Lucilia hypoleuca Wedd. ex Schultz-Bip, nom. nud.) Bolivia, Larecaja, Mandon 179 (holotype, S, isotype, NY).
Luciliocline longifolia (Cuatrec. & Aristeg.) M.O.Dillon & Sagást., comb. nov.
Lucilia longifolia Cuatrec. & Aristeg., Fl. Venezuela 10: 367. 1964. TYPE; Venezuela, Edo. Mérida, camino a Pico Bolívar, 15 km al sudeste de Mérida, E.L. Little 15725 (holotype, VEN). Belloa longifolia (Cuatrec. & Aristeg.) Sagást. & M.O.Dillon, Phytologia 58: 396. 1985.
Luciliocline pickeringii (A. Gray) M.O.Dillon & Sagást., comb. nov.
Lucilia pickeringii A.Gray, Proc. Amer. Acad. Arts 5: 138.1862. TYPE: Peru, Dept. Junín, Prov. Yaulí, Baños-Alpamarca, Capt. Wilkes s.n. (holotype, GH; isotype: US). Belloa pickeringii (A.Gray) Sagást. & M.O.Dillon, Phytologia 58: 396. 1985.
Luciliocline piptolepis (Wedd.) M.O.Dillon & Sagást., comb. nov.
Merope piptolepis Wedd., Chlor. And. 1: 162. 1856. SYNTYPES: Peru, Dept. Puno, Maravillas, H.A. Weddell 4514 (lectotype, P, designated by Cabrera, 1978). Belloa piptolepis (Wedd.) Cabr., Bol. Soc. Argent. Bot. 7: 81. 1958. Gnaphalium piptolepis (Wedd.) Griseb., Abh. Königl. Ges. Wiss. Göttingen. 24: 186. 1879.
Luciliocline plicatifolia (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov.
Belloa plicatifolia Sagást. & M.O.Dillon, Phytologia 58: 394. 1985. TYPE: Peru, Dept. Cajamarca, Prov. Contumazá, Cascabamba, arriba de Contumazá, A. Sagástegui A., E. García A., S. López M. & J. Mostacero L. 10117 (holotype, HUT; isotypes, F, HUT, K, MO). Lucilia plicatifolia (Sagást. & M.O.Dillon) S.E.Freire, Darwiniana, 28: 411. 1987.
Luciliocline radians (Benth.) M.O.Dillon & Sagást., comb. nov.
Gnaphalium radians Benth., Pl. Hartweg.: 207, t. 35b. 1845. TYPE: Colombia, , Prov. Popayan, prope Laguna de Guanacas, Hartweg 1146 (holotype, K). Lucilia radians (Benth.) Cuatrec., Trab. Mus. Cienc. Nat. Madrid, Ser. Bot. 33: 138. 1936. Belloa radians (Benth.) Sagást. & M.O.Dillon, Phytologia 58: 396. 1985.
Luciliocline schultzii (Wedd.). M.O.Dillon & Sagást., comb. nov.
Merope schultzii Wedd., Chlor. And. 1: 163. 1856. TYPE: Peru, Dept. Puno, Prov. Carabaya, Ayapata, W. Lechler 1984 (holotype, P, F neg 37608). Belloa schultzii (Wedd.) Cabrera, Revista Invest. Agríc. 11: 404. 1957.
Luciliocline spathulifolia (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov.
Belloa spathulifolia Sagást. & M.O.Dillon, Phytologia 58: 394. 1985. TYPE: Peru, Dept. La Libertad, Prov. Santiago de Chuco, entre Chota Motil y Shorey, A. Sagástegui A., J. Mostacero L., M. Diestra Q 11695 (holotype, HUT; isotype, F, MO, NY).
Luciliocline turnerii (Sagást. & M.O.Dillon) M.O.Dillon & Sagást., comb. nov.
Belloa turnerii Sagást. & M.O.Dillon, Phytologia 58: 392. 1985. TYPE: Peru, Dept. Cajamarca, Prov. Contumazá, Pozo Kuán, A. Sagástegui A., E. García A., S. López M. & J. Mostacero L. 10087 (holotype, HUT; isotype, F, HUT, MO, TEX).
Acknowledgements
I thank Billie L. Turner for the fable quoted at the beginning of this paper, for it in part has shaped my thinking in this group; do not overlook the obvious in classification. I thank Abundio Sagástegui Alva for companionship during field studies, providing much original material, and discussions on generic limits in Andean genera. Mary Reynolds, Betty Strack and Ron Wibel provided assistance with the original SEM studies. The American Society of Plant Taxonomy is thanked for partial financial support to attend the 1994 Kew Compositae Conference. Fred Barrie, Nancy Hensold, and Lúcia Kawasaki are acknowledged for reading various drafts of this paper and making valuable comments. Randall Bayer, Ilse Breitwieser, Guy Nesom, and Josephine Ward have all sharing unpublished data and shared their thoughts on various aspects Gnaphalieae classification and phylogeny. I thank the curators and collection managers of various herbaria where material was studied or borrowed, including CPUN, COL, GH, HUT, MO, NY, TEX, US, and USM. Finally, Mario Zapata Cruz is thanked for providing help with the Spanish translation.
References
Anderberg, A. A. 1991. Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Bot. 104: 1-195. ________.
1994. Tribe Gnaphalieae. In K. Bremer, Asteraceae, Cladistics and Classification, pp. 304-364. Timber Press, Portland, Oregon.
________. & S. Freire. 1990. Jalcophila boliviensis, a new species of South American Asteraceae (Gnaphalieae). Brittonia 42: 138-141.
________.& ________. 1991. A cladistic and biogeographic analysis of the Lucilia group (Asteraceae, Gnaphalieae). J. Linn. Soc. Bot. 106: 173-198.
Bayer, R., I. Breitwieser, M. Dillon, M. Koekemoer, & J. Ward. 2003. Phylogeny of the Gnaphalieae based on three cpDNA sequences (matK, trnL intron, and trnL/trnF intergenic spacer). Compositae Newsletter 40.
Bayer, R., I. Breitwieser, J. Ward, & C. Puttock. xxxx. Gnaphalieae (Asteraceae) Pages xx-xx. In: K. Kubitzki (ed.), The Families and Genera of Vascular Plants. Vol. Xx. Springer Verlag, Berlin, in press.
Bremer, K. 1994. Asteraceae, Cladistics and Classification. Timber Press, Portland, Oregon. 752 pp.
Cabrera, A. L. 1958. El género Belloa Remy. Bol. Soc. Argent. Bot. 7: 79-85.
________. 1961. Observaciónes sobre las Inuleae-Gnaphalineae (Compositae) de América del sur. Bol. Soc. Argent. Bot. 9: 359-386.
________. 1971. Compositae. Flora Patagónica. Colecc. Cient., Inst. Natl. Tecn. Agropec. 8(7): 1-451.
Cronquist, A. 1950. A review of the genus Psilocarphus. Res. Stud. State Coll. Wash. 18(2): 71-89.
DeCandolle, A. P. 1838. Lucilia. Prodr. 7(1): 46.
Díaz-Piedrahita, S., & C. Vélez-Nauer. 1999. Los géneros Jalcophila y Chevreulia (Asteraceae-Inuleae) in Colombia. Rev. Acad. Colomb. Cienc. 19(72): 25-26.
Dillon, M. O. 1988. Generic limits and microcharacters in the South American Gnaphaliinae (Asteraceae-Inuleae). Amer. J. Bot. Abst. 75: 456.
________. 1990. A tale of two genera: the implication of character choices and outgroup selection in cladistic analysis. Amer. J. Bot. Abst. 77: 129.
________. 2000. Classification and phylogeny of the South American Gnaphalieae (Asteraceae). Andean Botanical Information System. URL: www.sacha.org/Gnaphalieae/ Gnaphalieae.htm.
________. & A. Sagástegui A. 1986. Jalcophila, a new genus of Andean Inuleae (Asteraceae). Brittonia 38: 162-167.
________. & ________. 1990. Oligandra Less. revisited and the need for a new genus, Pseudoligandra (Asteraceae: Inuleae). Taxon 39: 125-128.
________. & ________. 1991a. Sinopsis de los géneros de Gnaphaliinae (Asteraceae-Inuleae) de Sudamérica. Arnaldoa 1: 5-91.
________. & ________. 1991b. Family Asteraceae: Part V. Tribe Inuleae. In, J. F. Macbride & Collaborators, Flora of Peru, Fieldiana: Botany, N.S. 26, 1-70.
Drury, D. G. 1970. A fresh approach to the classification of the genus Gnaphalium with particular reference to the species present in New Zealand (Inuleae-Compositae). New Zealand J. Bot. 8: 222-248.
________. 1971. The American spicate cudweeds adventive to New Zealand. New Zealand J. Bot. 9: 157-185.
Freire, S. E. 1986. Revisión del género Lucilia (Compositae, Inuleae). Darwiniana 27: 431-490.
________. 1987. A cladistic analysis of Lucilia Cass. (Compositae, Inuleae). Cladistics 3: 254-272.
________. 1993. A revision of Chionolaena (Compositae, Gnaphalieae). Ann. Missouri Bot. Gard. 80: 397-438.
Freire, S. E. & L. Iharlegui. 1997. Sinopsis preliminar del género Gamochaeta (Asteraceae, Gnaphalieae). Bol. Soc. Argent. Bot. 33: 23-35.
Hess, R. 1938. Vergleichend Untersuchungen ¸ber die Zwillingshaare der Compositen. Bot. Jahrb. Syst. 68: 435-496.
Holub, J. 1976. Gamochaeta. In, Tutin, T. G., et al. (eds.), Flora Europaea 4: 127.
Hooker, W. J. & G. A. W. Arnott. 1835. Contributions towards a Flora of South America and the Islands of the Pacific. Comp. Bot. Mag. 1: 102-111.
Morefield, J. D. 1992. Evolution and systematics of Stylocline (Asteraceae: Inuleae). unpubl. diss., 73 pps.
Merxmüller, H., P. Leins & H. Roessler. 1977. Inuleae--Systematic review. In V. H. Heywood et al. (eds.), The Biology and Chemistry of the Compositae, pp. 577-602. Academic Press, London.
Nesom, G. L. 1990a. Taxonomy of Gnaphaliothamnus (Asteraceae: Inuleae). Phytologia 68: 366-381.
________. 1990b. An additional species of Gnaphaliothamnus (Asteraceae: Inuleae) and further evidence for the integrity of the genus. Phytologia, 69: 1-3.
________. 1994. Comments on Gnaphaliothamnus (Asteraceae: Inuleae). Phytologia 76: 185-191.
________. 2001. New combinations in Chionolaena (Asteraceae: Gnaphalieae). Sida 19: 849-852.
Remy, J. 1847. Compositae. In C. Gay, Historia Física y Política de Chile, Botánica 3: 257-482.
Swofford, D. L. 1991. Phylogenetic Analysis Using Parsimony (PAUP), version 3.0s. Illinois Natural History Survey, Champaign.
Comparison of Generic Composition of Belloa, Lucilia, and Luciliocline
| Genus | fide Anderberg & Freire (1991) | fide Dillon (2003) |
|---|---|---|
| Belloa Remy
in Gay Type: Lucilia chilensis Hook. & Arn. [=Belloa chilensis (Hook. & Arn.) Remy in Gay] |
Belloa chilensis
(Hook. & Arn.) Remy in Gay B. kunthiana (DC.) Anderb. & S.E.Freire B. lehmannii (Hieron.) Anderb. & S.E.Freire B. longifolia (Cuatrec & Aristeg.) Sagást. & M.O.Dillon B. pickeringii (A.Gray) Sagást. & M.O.Dillon B. piptolepis (Wedd.) Cabrera B. plicatifolia Sagást. & M.O.Dillon B. radians Sagást. & M.O.Dillon B. schultzii (Wedd.) Cabrera |
Belloa chilensis (Hook. & Arn.) Remy in Gay |
| Luciliocline
Anderb. & S.E. Freire Type: Belloa lopezmirande Cabrera [= Luciliocline lopezmirande (Cabrera) Anderb. & S.E.Freire] |
L. burkartii
(Cabrera) Anderb. & S.E.Freire L. catamarcensis (Cabrera) Anderb. & S.E.Freire L. lopezmirandae (Cabrera) Anderb. & S.E.Freire L. santanica (Cabrera) Anderb. & S.E.Freire L. subspicata (Wedd.) Anderb. & S.E.Freire |
L. burkartii (Cabrera)
Anderb. & S.E.Freire L. catamarcensis (Cabrera) Anderb. & S.E.Freire L. longifolia (Cuatrec. & Aristeg.) comb. nov. L. lopezmirandae (Cabrera) Anderb. & S.E.Freire L. pickeringii (A.Gray) comb. nov. L. piptolepsis (Wedd.) comb. nov. L. plicatifolia (Sagást. & M.O.Dillon) comb. nov. L. radians (Benth.) comb. nov. L. schultzii (Wedd.) comb. nov. L. spathulifolia (Sagást. & M.O.Dillon) comb. nov. L. subspicata (Wedd.) Anderb. & S.E.Freire L. turnerii (Sagást. & M.O.Dillon) comb. nov. |
| LuciliaCass. Type: Serratula acutifolia Poir. [=Lucilia acutifolia (Poir.) Cass.] |
L. acutifolia
(Poir.) Cass. L. eriophora Remy L. ferruginea Bak. L. linearifolia Bak. L. lycopodioides (Less.) S.E.Freire L. nitens Less. L. recurvata Wedd. L. tomentosa Wedd. |
L. acutifolia
(Poir.) Cass. L. araucana Phil. L. conoidea Wedd. L. eriophora Remy L. ferruginea Bak. L. kunthiana (DC.) Zardini L. linearifolia Bak. L. lycopodioides (Less.) Freire L. nitens Less. L. nivea (Phil.) Cabrera L. recurvata Wedd. L. tomentosa Wedd. |
Figures
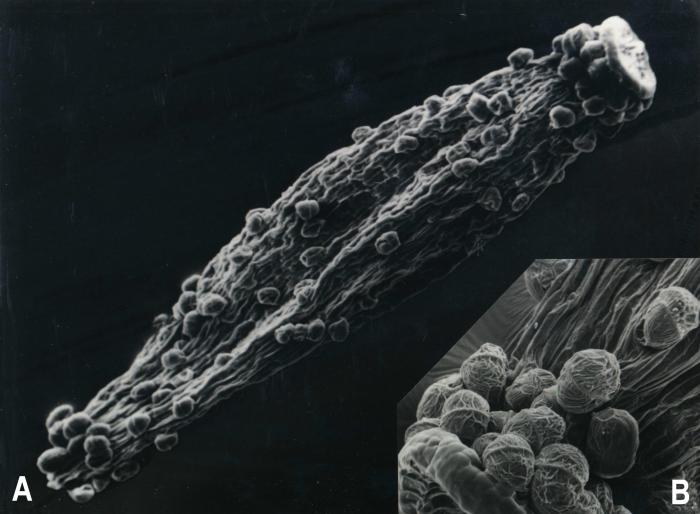
Figure 1. Luciliocline longifolia. A. Achene [1280 µm long]. B. Trichomes amplified. (Voucher: Sagástegui et al. 12841, F).
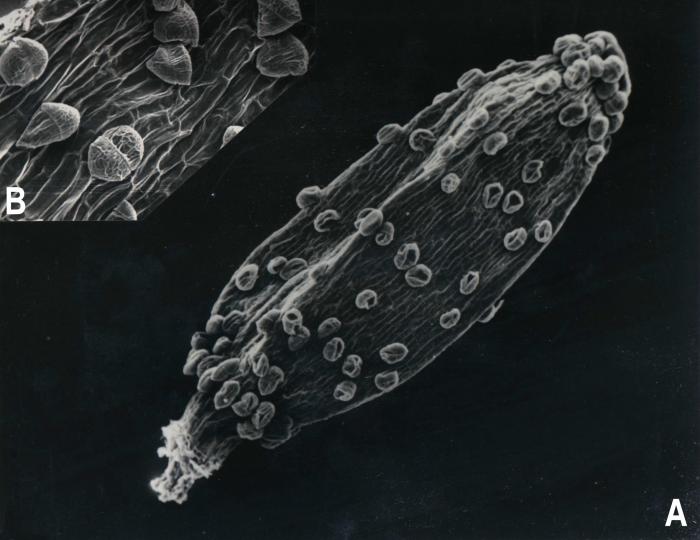
Figure 2. Luciliocline piptolepsis. A. Achene [710 µm long]. B. Trichomes amplified. (Voucher: Sagástegui et al. 12658, F).
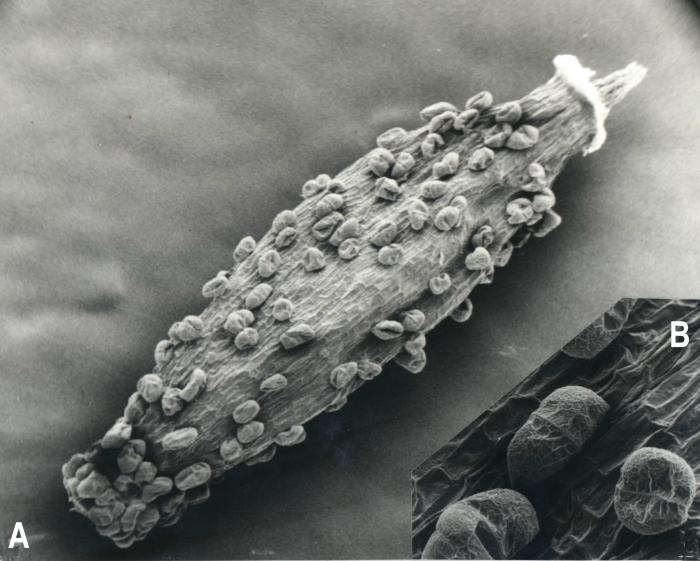
Figure 3. Luciliocline plicatifolia. A. Achene [1360 µm long]. B. Trichomes amplified. (Voucher: Sagástegui et al. 10719, F). 3

Figure 4. Belloa chilensis. A. Achene [1150 µm long]. B. Trichomes amplified. (Voucher: Teillier et al. 2020, F).